
Osimertinib capsule
Generic name: Osiinda
Product name: Osimertinib capsule
Preparation: Capsule
Specification: 250mg/30capsule
Product
Osimertinib, sold under the brand name Osiinda is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.Osimertinib was approved for medical use in the United States in November 2015,and in the European Union in February 2016.Osimertinib is used to treat locally advanced or metastatic non-small-cell lung cancer (NSCLC), if the cancer cells are positive for the T790M mutation in the gene coding for EGFR or for activating EGFR mutations. The T790M mutation may be de novo or acquired following first-line treatment with other tyrosine kinase inhibitors (TKIs), such as gefitinib and afatinib.
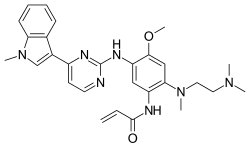
In the US, EGFR exon 19 deletions, exon 21 L858R mutations or the T790M status of the patient prior to treatment with osimertinib must be detected by a federally approved companion diagnostic test. The Food and Drug Administration (FDA) has approved FoundationOne CDx as one available companion diagnostic test for this purpose. In Europe and elsewhere, activating EGFR mutations or T790M mutations may be determined by a validated test.In people treated with osimertinib, resistance usually develops within approximately 10 months. Resistance mediated by an exon 20 C797S mutation accounts for the majority of resistance cases.

INDAR (INDAR) has launched more than 600 kinds of drugs, including conventional oral tablets, capsules, suspensions, eye drops and injectable biological products. Advanced production line, clean GMP plant and strict quality control have won GMP certification from drug regulatory authorities of many countries, including MHRA (British drug administration) and EU (European Union). Its products are exported to all parts of the world, including the UK, EU Member States and many developing countries.







